Yes, there are mites living on your face — and they can poop, a new study finds
Our first look at their genome reveals new details of their microscopic lives
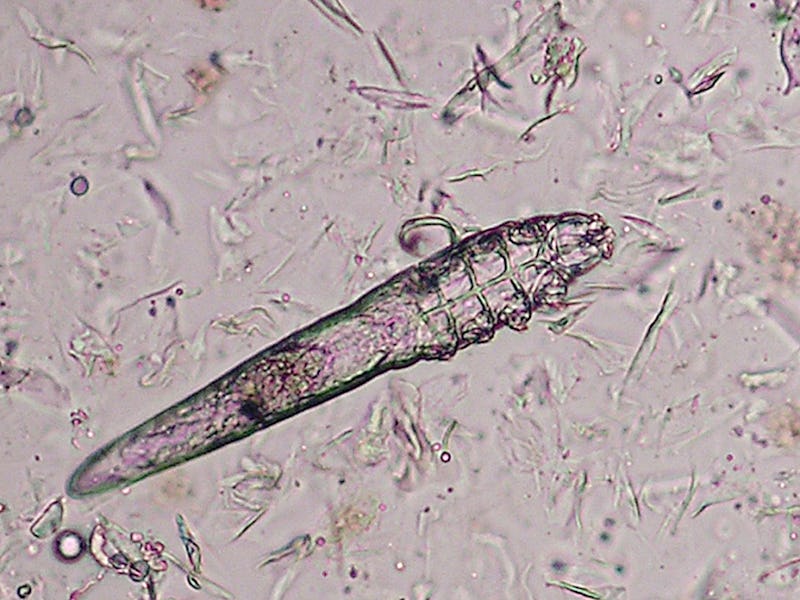
If you’re arachnophobic, it’s best to stop reading. This isn’t about spiders, but another type of arachnid — the microscopic ones living in the hair follicles on your face. Yes, I’m talking about the mites that naturally live inside your pores. No, I’m not lying to you.
Despite how they look under the microscope, human follicular mites, a.k.a. Demodex, are fairly ubiquitous and mostly harmless if not beneficial. In fact, they are present on nearly every human, typically around the eyelashes and nose, and help keep our pores clear by eating dead skin cells and natural oils. (Someone has to do it.) They only cause skin disease when present in large quantities.
Demodex folliculorum may look like a CGI sci-fi monster under a microscope, but it (probably) won’t hurt you.
From egg to larva to full-grown adult, these eight-legged, semi-transparent creatures live only a few weeks. They spend their lives eating, mating, and laying eggs, all inside your hair follicles, from which they only emerge at night.
Humans have a fairly intimate relationship with Demodex. Despite this, scientists know relatively little about these tiny creatures. Now, for the first time, researchers have sequenced the genome of the most common species of Demodex in humans, Demodex folliculorum, providing a new window into their microscopic lives — and the evolutionary dead-end toward which they might be headed.
What’s new — In a paper published on Tuesday, June 21 in Molecular Biology and Evolution, researchers dug into the DNA of these follicular mites and found a trove of information about their anatomy, behavior, and evolution that scientists had previously only suspected.
“This provides all Demodex researchers around the world with much-needed information on their genetic make-up,” says Benjamin Clanner-Engelshofen, who researches the mite at the LMU Clinic of the Ludwig Maximilian University of Munich. He was not involved in the recent study, though his research team is also working to sequence Demodex’s genome.
The process of extracting the DNA was challenging, especially because the researchers had to clean hundreds of mites by hand using tiny tools to prepare for the extraction.
“They are so small and fragile,” says Alejandra Perotti, one of the authors of the new study, tells Inverse. Perotti studies invertebrate biology at the University of Reading in the UK. “Being so microscopic makes them elusive.”
What they found — The results were worth the toil, revealing details of the elusive mites’ lives. For example, scientists knew that the male Demodex’s penis, also called the aedeagus, is in an unusual location: on its back, pointing toward its head. The researchers found that a group of genes that determine where and how different body parts develop, called Hox genes, had been shuffled around from their usual order in other animals. This could explain the unique positioning of the Demodex penis, the authors suggest.
Demodex might have the weirdest penis you’ve seen this week (circled in white).
And then there’s the anus. Scientists previously thought that Demodex did not have an anal opening and that they expelled their waste into the follicle at the end of their short lives. The authors identified the opening under a microscope and found genes that would indicate the presence of an anus — confirming that Demodex can, in fact, poop before they die.
But it’s details of the mite’s nocturnal lifestyle that truly wowed the researchers. Scientists have known that Demodex emerges from its follicular home to reproduce while we sleep. The mite’s inverse schedule could be explained by the hormone melatonin, which helps humans sleep but has the opposite effect on invertebrates like Demodex, stimulating them to move and mate.
To explain this, the researchers looked at the Demodex DNA and found that the mites lack a few key genes for maintaining a biological clock and producing melatonin. The authors came to a surprising conclusion based on this new information: The mites may be using their human hosts’ melatonin to cue and fuel their nighttime mating adventures.
Demodex mites are so small — around a third of a millimeter long — that they can only be seen under a microscope. Their eight stubby legs allow them to travel about one centimeter each hour across your face.
The big picture — These mites have been members of our body’s ecosystem since before modern humans evolved. Despite their size, they are just as much a member of the animal kingdom as we are. But compared to other arachnids like ticks, fleas, and different species of mites, Demodex are not very complex organisms — they have the lowest number of genes out of fifteen arachnid species the authors surveyed.
The authors conclude that Demodex has been losing genes over time, evolving to be less complex by relying more on their hosts. Part of this may be thanks to isolation and inbreeding, since mite lineages mainly encounter, and therefore reproduce with, other members of their same genetic line, which can erode the genome.
All of this indicates to the researchers that D. folliculorum may be headed toward an evolutionary dead-end and eventual extinction — though they seem to be doing just fine for now. It’s unclear what effect this eventuality would have on us, their human hosts.
What’s next — Now that scientists have sequenced the mite’s genome, they may be able to develop better treatments for the skin conditions that can arise when Demodex runs amok, says Clanner-Engelshofen. These conditions, like demodicosis and blepharitis, usually affect older adults and people with compromised immune systems and are currently treated with creams like the infamous ivermectin that kill the mites. This new genetic information could be used to develop better treatments that target and kill the mites’ larvae and present little to no adverse effects for the patient, Clanner-Engelshofen says.
And while these results are an exciting first step, they represent the genome of only one mite from one lineage. People living in different areas of the world are home to different lineages of Demodex mites, according to Michael Palopoli, an evolutionary biologist at Bowdoin College who has published research on Demodex’s evolution. Genetic sequencing would allow scientists to tease apart the worldwide differences between these mites, he says, and to investigate how they might affect human health.