3D brain model cracks open a mystery of human development
The new stem-cell models provide our first glimpse of previously unseen neural dynamics.
Some say that out of all the objects in the universe, the human brain is the most complex. Much of what makes us uniquely human — our emotions, memories, consciousness — stems from this 3-pound organ, yet the bulk of what we know about the brain comes from studying animals.
In a landmark new study, researchers have recreated the human forebrain using 3D brain organoids — tiny cellular models of the brain — that they say successfully model late stages of brain development for the first time. The model opens a window onto the development of the human forebrain, which plays a key role in the processing of information related to complex cognitive activities, sensory processing, and motor function.
This isn't the first foray into growing 3D stem-cell structures that mimic brain development, but what sets the new organoids apart is that they map onto key brain regions in humans, enabling researchers to understand how the human brain develops — and what happens when it goes awry. They are also able to be kept for up to 600 days — meaning researchers can study how they change over a long period of time, bringing us closer to understanding how our own brains develop.
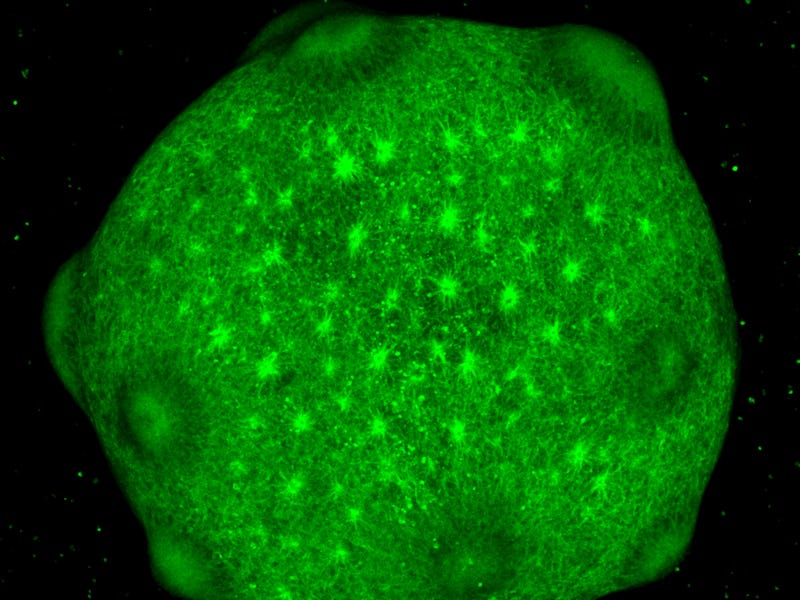
This surreal image shows the brain organoids in culture — the organoids survive for up to 600 days, researchers say.
The method clears a massive hurdle in neuroscience research — namely, being able to study the molecular processes that underlie brain development and function in humans over the course of time. Until recently, much of what we know about how the brain develops has been limited to studying similar processes in animal models and post-mortem human brains. The organoids could become vital tools for scientists around the globe as they go after questions about the brain, previously out of reach.
“Lab grown human brain organoids can be maintained in a dish for hundreds of days, and by recapitulating human brain development they could serve as a powerful model to study disease,” Sergiu Pasça, co-author of the study and researcher at Stanford University, tells Inverse.
Pasca and his team published their model on Thursday in the journal Science.
Mapping models to brains
To generate the models, the researchers took a clump of “human induced pluripotent stem cells,” and moved them to a petri dish that is prepared so that the clump of cells “can’t sit down,” Pasça says. These cells can become any type of cell in the body, but the team used drugs and growth factors to signal the cells to eventually develop into cells mimicking those of the human forebrain.
“These organoids are floating, spherical structures that self-organize and, as we have shown, can be kept in a dish for very long periods of time — up to 600 days in this study,” Pasça says.
That the organoids can be maintained over long periods of time solves another persistent problem in neuroscience research: Studies that rely on human brain tissue generally use post-mortem brain tissue, but this tissue degrades rapidly after death, within a few days. That means it may not be the most reliable sample for a study. And, of course, it’s not currently possible to study the brain of an embryo in utero at this resolution, although it is now possible to analyze embryos' brain activity through MRIs, EEGs and other tests.
Human brain organoids grown in cellular cultures in the lab.
To demonstrate the utility of their model, the researchers created a map of chromatin accessibility — chromatin is the part of the chromosome that contains DNA, RNA and protein. Chromatin acts like the brain and body’s drill sergeant, dictating how genes express and, in turn, how the brain will develop.
When they compared their organoids to real human tissue, they found that the models accurately corresponded with different regions of the forebrain. Taken together, the organoids and chromatin map provide a glimpse into how the brain changes at different stages of development.
The forebrain includes the thalalumus, epithalamus, hypothalamus, and subthalamus — these brain regions play a role in the development of some of our most cherished traits, including human speech, abstract thought, and various bodily functions like sex drive, blood pressure, and hunger.
“The forebrain ultimately gives rise to multiple brain regions, including the cerebral cortex, which is the most expanded brain region in humans and responsible for higher brain functions, such as cognition and language,” Pasça says.
By modeling the forebrain, scientists can get a clear glimpse of one of the most crucial periods of human development.
Previously, scientists hadn't been able to study how the forebrain forms closely, in part because the assembly of the human forebrain takes a long time in development, Pasça says.
“Billions of cells are generated in a very well-coordinated order. Disruption, either by environmental factors — infections — or genetic factors — mutations — can lead to severe neurodevelopmental disorders such as autism, schizophrenia, or epilepsy,” Pasça says.
New pathways to treatment
Drilling down on what goes wrong, and when, is vital for the estimated 450 million people who have these conditions.
The research is preliminary, but the organoids offer potentially powerful disease models for neuroscientists everywhere, he says.
For example, Pasça and his team used the organoids to capture previously inaccessible stages of human brain development and mapped genetic risk for autism and schizophrenia in specific cell types and time points. Down the line, the organoids could help researchers develop screenings that target genetic markers for these and other neuropsychiatric conditions, like depression, enabling their diagnosis — and even treatment — long before the condition ever manifests in outward symptoms.
As a next step, Pasça and his team are studying 3D brain-organoid models derived from individuals that have neurodevelopmental and neuropsychiatric disorders.
“By comparing these side by side with unaffected patients, we can gain even more insights into the origins of these complex diseases," he says.
Abstract: Forebrain development is characterized by highly synchronized cellular processes, which, if perturbed, can cause disease. To chart the regulatory activity underlying these events, we generated a map of accessible chromatin in human three-dimensional forebrain organoids. To capture corticogenesis, we sampled glial and neuronal lineages from dorsal or ventral forebrain organoids over 20 months in vitro. Active chromatin regions identified in human primary brain tissue were observed in organoids at different developmental stages. We used this resource to map genetic risk for disease and to explore evolutionary conservation. Moreover, we integrated chromatin accessibility with transcriptomics to identify putative enhancer-gene linkages and transcription factors that regulate human corticogenesis. Overall, this platform brings insights into gene-regulatory dynamics at previously inaccessible stages of human forebrain development, including signatures of neuropsychiatric disorders.