The next promising cancer treatment also makes your lungs glow
This could help make smaller tumors easier to spot and remove.
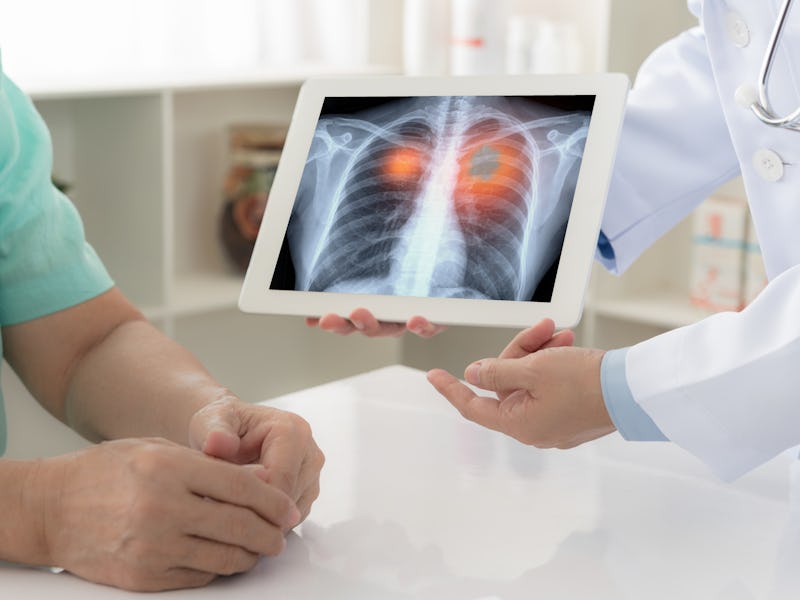
In cancer treatment, being able to identify and fully remove tumors is a front line of defense against disease progression and relapse. But, some tumors are so small that they evade the notice of physicians. To help shine a brighter light on these elusive tumors, scientists have designed a drug to make them glow in the near-infrared.
Lung cancer is among the world's most common and deadly cancers, with the American Cancer Society estimating a staggering 228,820 new cases of the disease in the United States this year alone. While smoking is a leading cause of lung cancer, the cancer can also develop from exposure to pollution or other harmful environmental chemicals as well as through genetic disposition. To support the fight against this common cancer, a team of researchers developed a drug that would enable better identification of these tumors without patients needing an invasive thoracotomy.
University of Pittsburgh Medical Center thoracic surgeon and co-author on the study, Inderpal (Netu) Sarkaria, said in a statement that their drug, not-so-catchingly dubbed OTL38, could improve patient outcomes through better quality of care.
"Lung cancer is the most common and lethal cancer worldwide," said Sarkaria. "Technologies to improve the care of these patients are needed. Near-infrared imaging with OTL38 during surgery for lung cancer is one such promising technology with the potential to significantly improve the completeness and quality of the operation, therefore improving patient outcomes."
The research, which was presented on Monday at the 56th Annual Meeting of The Society of Thoracic Surgeons in New Orleans, Louisiana, was conducted as part of a phase two clinical trial with 92 patients who were already slated to undergo pulmonary resection (e.g. surgery to remove tumors from the lungs) for the most common type of lung cancer: non-small cell lung cancer (NSCLC.) Before undergoing their surgeries, the patients were given an intravenous injection of the drug which contained near-infrared, fluorescent dye and a targeting molecule designed to attach itself to cancer cells in the patient's lungs, making it easier for surgeons to spot during the operation using a special surgical endoscope.
While surgeons typically use x-rays and MRIs to identify tumor location and size before surgery, a benefit of this approach is that it can still be used during surgery to help identify tumor growth that other methods may have missed.
When evaluating the success of the drug post-operation, the team of researchers found that in 12 percent (11 out of 92) of patients surgeons were able to remove legions in the lungs that had not been identified prior to the operation. However, when inspecting the legions, the team also found that small amounts of the tumors identified by the target molecule were still leftover after operation in 9 percent (8 out of 92) patients, meaning that the targeting molecule was not totally successful in identifying and fluorescing entire tumors. With these mixed results in mind, the team determined that the drug was successful in improving surgical outcomes in 26 percent (roughly 1 in 4) of the trial patients.
"Use of advanced near-infrared imaging techniques such as OTL38 may provide surgeons with powerful tools to improve the quality of lung cancer operations by better identifying small, hard-to-find tumors, finding previously undetected cancers at the time of surgery, and better assessing if the entire tumor has been removed," said Sarkaria.
While this drug does appear to show promise when it comes to better identify overlooked clusters of cancer cells, there's still a long road ahead before it ever reaches a patient. The research is now moving on to its phase 3 clinical trial (which will look at a wider patient group of a few thousand participants) but still has to complete FDA approval and an even larger still phase 4 clinical trial. While these next phases of clinical testing are not impossible hurdles, they are no trivial feat either.
Abstract: COMMERCIAL RELATIONSHIPS Inderpal S. Sarkaria Speakers Bureau/Honoraria: Intuitive Surgical Speaking/teaching engagements with honoraria Consultant/Advisory Board: Cambridge Medical Robotics (CMR) Advisory and consulting regarding product development REGULATORY DISCLOSURE Drug FDA approval?: Yes Product FDA approval?: No Type: Drug Status: Investigational Name of device/product/drug: OTL-38 Company that owns device/product/drug: On Target Laboratories Off-Label Use details: This presentation describes the off-label use of OTL-38, an investigational drug approved for this clinical trial as a novel drug for intraoperative molecular imaging.
Purpose: Intraoperative molecular imaging (IMI) is an intraoperative tool that may improve surgical outcomes for NSCLC. A multi-institutional Phase 2 clinical trial involving a near-infrared, folate-receptor targeted agent (OTL38) was conducted.
Methods: Patients with suspicious lung lesions received 0.025 mg/kg of OTL38 before surgery. The primary goal was to determine if OTL38 improved surgeons’ ability to localize hard-to-find lesions, identify occult cancers, and discriminate close margins. Assessments were made in three phases: (i) “Lung Inspection Phase” in which the hemithorax was evaluated for other pathology, (ii) “Tumor Resection Phase” in which the primary tumor was resected, and (iii) the “Specimen Check Phase” in which the resected specimen was assessed on the back table for margin status. All specimens were verified by two blinded pathologists and stained by immunohistochemistry for folate receptor expression.
Results: 110 patients were recruited; 92 were eligible for analysis. There were no drug-related serious adverse events. During the Inspection Phase, additional lesions were found in two patients using standard-of-care visual inspection and manual palpation alone, while ten additional cancers were found in 7 patients (8%) by IMI. During the Resection Phase, IMI enabled localization of otherwise unlocalizable lesions in 11 (12%) patients. During the Specimen Check Phase, surgeons felt all margins were adequate, however, back-table IMI revealed inadequate margins in 8 patients (9%). The IMI learning curve was 6 cases. Benefits of IMI were most profound in patients undergoing sublobar pulmonary resections (wedge and segmentectomy) and in patients with ground-glass opacities.
Conclusions: In this Phase 2 clinical trial, IMI improved outcomes for 26% of patients undergoing pulmonary resection for NSCLC. This technology may be a useful adjunct during pulmonary resections. An Phase 3 clinical trial is being undertaken.