Spravato: Release Date, Cost, Ingredients for Ketamine-Based Nasal Anti-Depressant
Here's how it works, the potential risks, and how it will be used in clinics.
Ketamine, once saddled with a bad reputation as a ‘90s-era trance drug, has officially completed an FDA-approved rebrand for its ability to dramatically improve depression symptoms much faster than other treatments.
The clear nasal spray is called Spravato, and it’s manufactured by Johnson & Johnson. The FDA recommends Spravato for patients who have “treatment-resistant depression,” which means that they’ve tried at least two other treatment options to manage their condition without success. It’s estimated that one-third of people with depression have the variety that’s treatment-resistant.
On Tuesday, March 5, the FDA declared the ketamine-based nasal spray to be a safe and effective way to treat depression, and research shows the drug can be effective in hours instead of weeks. The results are credited to the use of esketamine, one of the two mirror-image molecules that form ketamine.
Numerous clinical trials suggest that estketamine holds great promise as a depression treatment — so much so that an FDA panel voted 14-2 to approve it in February. The FDA has now followed through on those recommendations, and patients with depression will soon get to try it out.
How Does Spravato Work?
Part of the reason that Spravato is making a splash is its primary ingredient. Esketamine is one of the two mirror-image molecules that make up its parent molecule, ketamine. (Ketamine is actually already FDA-approved as an anesthetic and is sold under the brand name Ketalar.) Ketamine, too, has shown promise as a treatment for depression, but ketamine’s recreational abuse potential made esketamine much more attractive to the FDA’s experts for approval as an anti-depressant.
Esketamine, related to the party drug ketamine, was recommended by an FDA panel for treating depression.
Spravato differs from previous treatments for depression on two fronts: For one, it works quickly — in hours instead of weeks. In a clinical trial published in The American Journal of Psychiatry in December 2017, 55 percent of patients who received ketamine treatments saw a 50 percent reduction in suicidal thoughts after just 24 hours. Only 30 percent of the control group saw a reduction in suicidal thoughts during that time period. By comparison, other anti-depressant drugs are intended to work over the course of weeks.
Spravato is also notable because it actually works differently from other anti-depressants. One common class of anti-depressants is selective serotonin reuptake inhibitors (e.g., Prozac). These drugs help the brain increase its levels of serotonin, a neurotransmitter with a reputation for effects on mood. Ketamine and esketamine, on the other hand, exert effects on a different neurotransmitter, called glutamate. That makes it a promising option for those whose depression isn’t treated by adjusting serotonin levels.
How Much Does Spravato Cost?
Like many prescription drugs, pricing for Spravato will be complicated. For patients, the cost will greatly depend on insurance coverage, how often they are treated, and what dosage they are given during each session.
In an email to Inverse, Greg Panico, a spokesperson for Johnson & Johnson, explains Spravato’s cost by talking about the “wholesale acquisition pricing.” That is the price clinics will pay to obtain the drug for their patients, assuming that they don’t get any rebates, government subsidies, or special deals from the drug company.
During the first month of treatment, patients will either receive a 56mg dose or an 84mg dose of the drug twice per week. The cost of Spravato per treatment session will range from $590 - $885 for clinics (again, at this point the out-of-pocket cost for patients is difficult to pin down). Panico adds that Spravato’s wholesale price is “generally comparable with other specialty mental health drugs, such as long-acting injectables for treatment of schizophrenia.”
How Do People Take Spravato?
Just because the FDA has given Spravato its stamp of approval, that doesn’t mean that there aren’t risks. Ketamine-based treatments are known to induce trance-like states, and in clinical trials, some patients who took Spravato reported side effects like disassociation, dizziness, sedation, decreased feeling or sensitivity, anxiety, lethargy, increased blood pressure, vomiting, and feeling drunk, according to the FDA’s release.
Ketamine has shown promise as a treatment for depression, and the FDA just approved a new ketamine-based drug to help combat it.
One study, published in August 2018 in the American Journal of Psychiatry went so far as to suggest ketamine is an opioid after scientists showed that it activated opioid receptors in the brain. Those authors suggest that “ketamine should be administered under a psychiatrist’s supervision and monitoring, and with plans to transition patients from ketamine to other medications or devices if the depression recurs.” In other words, you won’t be able to walk out the door of your doctor’s office with Spravato.
The FDA also agrees Spravato has potential for abuse. That’s why patients can only take it under the supervision of a clinician, who will literally watch them snort the nasal spray.
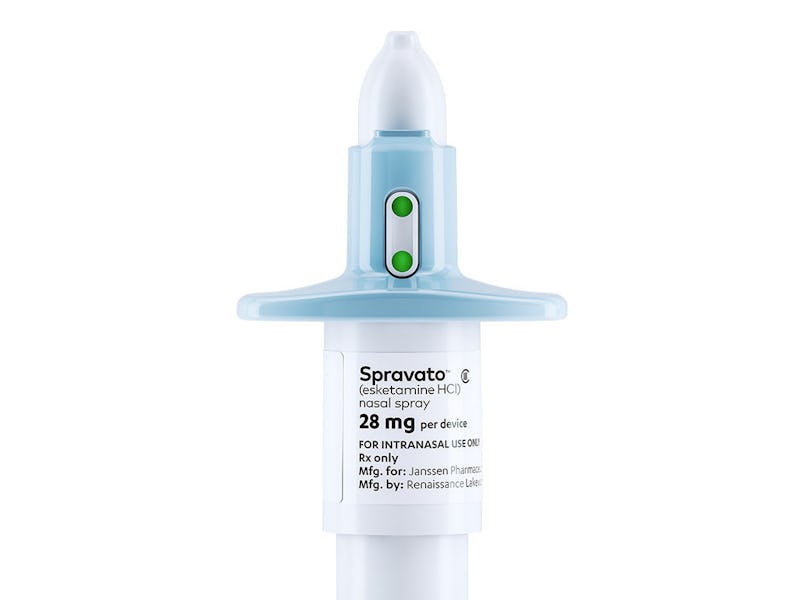
According to Johnson & Johnson’s prescriber information, the procedure will go something like this: A typical Spravato dose is two sprays from a device which contains a total 28mg of the drug. Depending on whether the patient is taking the 56 or 84 mg dose, the patient will either snort the contents of two or three devices. Johnson & Johnson’s recommendation also state that the physician should monitor patients for at least two hours after administering Spravato.
When Will Spravato Be Released?
Spartavo only received FDA approval on Tuesday, March 5, and because the drug can only be administered in a very specific way, it will only be available at certified locations. Panico explains that this approval process is ongoing, so there is no exact release date specified for Spravato, but that we can expect to see some information regarding which treatment centers will carry it soon.
“Later in March there will be a website where people will be able to track which sites are certified in their area,” he said.
Here’s a link to that website, which should be updated soon as sites gain approval to administer the drug. As of March 6, there are none listed so far.