Winner of Prestigious Science Photo Competition Shows Near-Impossible Shot
Look closely.
By the end of high school, most of us had some idea of what an atom is. They’re the most basic forms of the elements on the periodic table. They’re tiny spheres that theoretically make up everything in the universe, from the cells in our bodies to the air that we breathe. They can be split to create an atomic bomb. Sometimes they’re drawn like solar systems of round protons and orbiting electrons, though of course that’s not actually how they look. As with so many concepts in science, the tricky thing about atoms is that we can’t really see them.
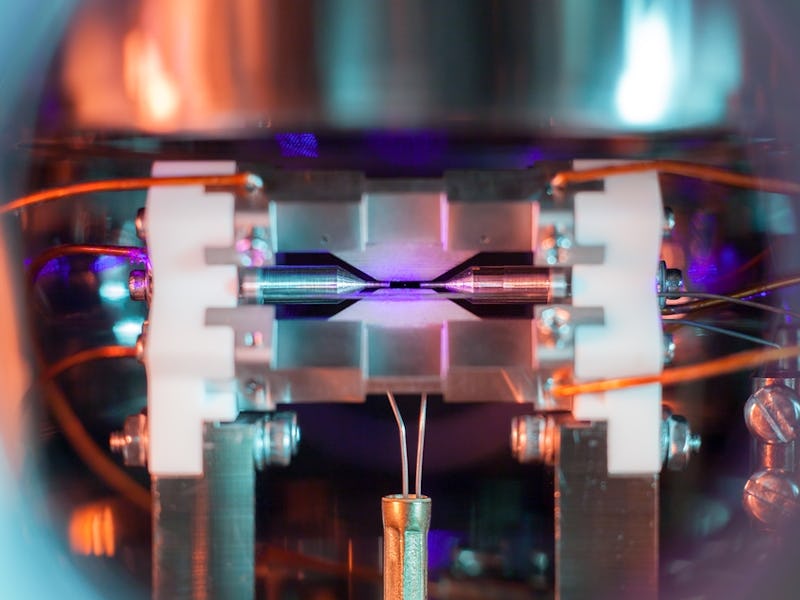
But the winning photo in a prestigious, U.K.-wide science photography competition organized by the nation’s Engineering and Physical Sciences Research Council (EPSRC) has changed that. The photo, taken by Oxford University quantum physicist David Nadlinger, Ph.D., is entitled ‘Single Atom in an Ion Trap.” The title is self explanatory: Nadlinger literally captured a photo of a single atom in a device called an ion trap. The closest scientists have come to doing this was when Griffith University researchers photographed the shadow of an atom in 2012.
But here’s Nadlinger’s atom, in all its minuscule glory. You might have to squint.
'Single Atom in an Ion Trap' by David Nadlinger.
Zooming into the space between the trap shows a slightly better view of the atom, which Nadlinger described with a reference to Carl Sagan’s timeless words about our planet.
“The idea of being able to see a single atom with the naked eye had struck me as a wonderfully direct and visceral bridge between the miniscule quantum world and our macroscopic reality,” Nadlinger said in an EPSRC statement. “A back-of-the-envelope calculation showed the numbers to be on my side, and when I set off to the lab with camera and tripods one quiet Sunday afternoon, I was rewarded with this particular picture of a small, pale blue dot.”
Nadlinger's "pale blue dot."
Ion traps are a family of devices that use magnetic and electric fields to capture individual charged particles (ions are just atoms that don’t have a stable number of electrons), which are useful to quantum physicists studying time and quantum computing. To prevent the atom from zooming off, the trap employs an ultra-high vacuum chamber. Nadlinger took this photo by pointing his camera through a window of this chamber, capturing the atom trapped in the 2-millimeter space between two needles.
The atom in this photo is a positively charged ion of strontium; when enough of these ions network together, they form what we know as the silvery metal strontium. But since we can only see things that reflect light, Nadlinger illuminated the atom with a specific blue-violet laser, which caused the atom to absorb and re-emit enough light for a long exposure photograph to capture.
A whole lot of strontium atoms connected to each other in crystal form.
Nadlinger’s photograph may not make it any easier for students of science to understand the quantum structure of the atom, which requires breaking it down to even tinier, impossible-to-photograph parts. But at least it provides our struggling brains with something tangible to work with.