Relax, Pig-Human Chimeras Have a 'Safety Switch' That Prevents Sentience
These pigs aren't going to be thinking human thoughts anytime soon.
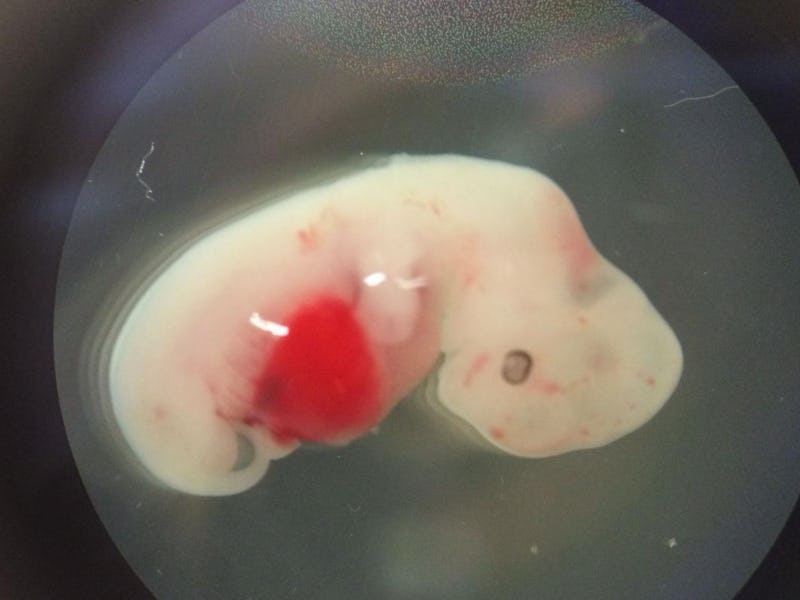
History’s first pig-human hybrids have been grown in a lab, stoking fears of a porcine mutant apocalypse. The strange hybrids, known as chimeras, contain a mixture of pig and human cells. Ethicists fear that the human cells, injected into a pig’s body with the intention of producing human organs, might travel to the brain and create a sentient pig with human thoughts.
It’s a terrifying scenario, but it’s also as unlikely as it is fantastical: The scientists behind the study want to reassure us that they’ve got a “safety switch.”
Calling from the Salk Institute in La Jolla, California, stem cell researcher Jun Wu, Ph.D., who worked on the study published this week in Cell, assured Inverse, “We already have the strategy.” He’s referring to a biological mechanism he and his colleagues, led by Pope Francis-approved chimera pioneer Juan Carlos Izpisua Belmonte, Ph.D., devised to prevent human cells from straying into parts of the body that could cause ethical concerns — the brain, in particular. In one method the Izpisua Belmonte lab has designed, Wu explains, cells that threaten to do so can be programmed to self destruct. They didn’t describe it in their paper because they hadn’t found evidence of any cells that had traveled to the brain in the 17 embryos they analyzed, and the fact that they terminated the embryos well before they came to term eliminated the possibility they could become sentient. But, Wu says, it’s a possibility they are preparing for.
“If we let the gestation go for longer, then we need to start to incorporate this safety switch,” he says.
Human stem cells were pipetted into a pig embryo.
In a chimera — the scientific term for a hybrid animal, named for the Greek lion-snake-goat hybrid — runaway cells don’t occur often, but they aren’t easy to detect or catch when they do. They’re created by growing the host animal — the pig, in this case — from the embryo stage in a dish; a little later, stem cells from the donor — that’s humans — is injected into it. It’s then given the time and resources to grow, while scientists watch. In the Cell study, Wu and his colleagues did this to figure out what type of human stem cells best survive inside a pig embryo (their conclusion: “intermediate” pluripotent stem cells).
In doing so, Wu and his colleagues brought science one step closer to creating pigs with human internal organs that we can someday farm for ourselves. In the future, keeping the human cells within the region of the intended organ will be crucial if Wu and his peers want to avoid moral backlash.
Their strategy for preventing stray cells is twofold. First, they use gene editing to scoop out a hole in the pig for the human cells to fill. If they wanted to grow a liver, they would engineer a pig that lacked one: Deleting the liver genes from the pig embryo at the one-cell stage results in a pig with a liver-shaped hole its organ should be. Human stem cells, which can be coaxed into turning into liver cells, will fill that hole when they’re injected into the pig embryo. There, “the human cells will outcompete the host cells to occupy the liver niche and generate the liver,” Wu explains. The idea of competition is central to keeping cells in place: It’s hoped that in other parts of the pig body, the pig’s cells will assert their priority over the human cells and shove them out.
But that’s not completely foolproof. That’s where phase two — engineering the human cells — comes in. “If we don’t want human cells to go to, for example, the brain, what we could do is we can modify the human cells genetically or epigenetically to prevent the human cell from going to the brain. Those two we can control,” Wu says. These modifications either avert the human stem cells from physically traveling to the brain — sort of like an ankle monitor for runaway cells — or stop them from developing into neurons. If either of these two events occur, Wu says, “automatically they will self destruct physically.”
Bebop from Teenage Mutant Ninja Turtles: also a human-pig chimera.
Armchair ethicists can let out a collective sigh of relief: the pigs will not become sentient. What’s even more comforting than the existence of the “safety switch” is knowing that chimera scientists are just as concerned about the morally sketchy possibilities of their work as everyone else. “People worry about a pig born with human cells in the brain,” Wu says. “But this is not what we tried to do here.” At this stage of the research, the work that goes on in the Izpizua Belmonte lab doesn’t require the pig embryos — transplanted into sows — to be born, so the possibility of pig consciousness remains remote for now.
But won’t those pig embryos have to come to term if we’re going to harvest their livers and hearts for ourselves? Wu explains that the idea of pigs-as-organ-farms is a bit misleading; for some organs, such as the pancreas, the fully grown organ won’t be necessary for transplantation — a culture of its progenitor cells will be enough. However, he admits, other organs like the heart do need to be fully grown, “but I think before that there are still a lot of challenges we have to overcome.”
Whether human cells that travel to a pig brain will even give rise to human consciousness in a pig remains unknown. Unfortunately for scientists and philosophers, there is only one way to find out. This is ethically murky territory: Wu and his colleagues are concerned, too, that human cells might integrate with a pig’s germline — that is, the cells that give rise to offspring. Again, the possibility is a long way off — but it’s only by imagining an apocalyptic future that the tools for preventing it can be developed in the present.